Skip to main content
Current Research
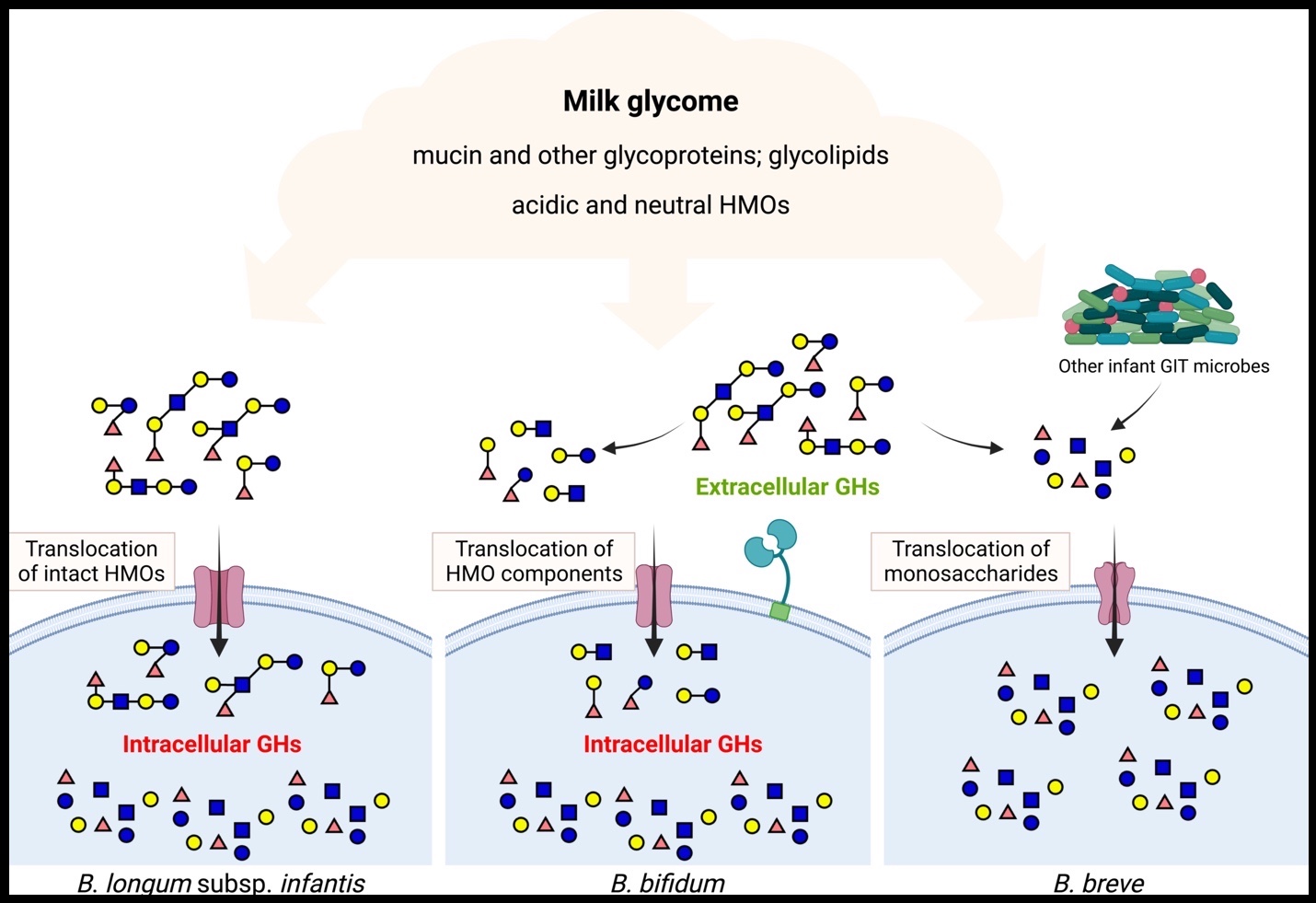
- Breast fed infants are typically colonized by bifidobacteria that are thought to protect, feed and communicate with the developing intestine. Human milk contains a large amount of free and bound glycans thought to be involved in this bifidobacterial enrichment and my research has focused on deciphering the molecular mechanisms underlying this glycan-based enrichment. Work from my lab has produced the one of most detailed maps of glycan-degradation by a commensal bacterium, providing a significant new model for host-microbe interactions. More importantly, this work provides a conceptual basis for manipulation of the gut microbiota via synbiotic applications of milk glycans and cognate bifidobacteria—applications with very near-term impacts on human health.
- Interactions between food glycans, the gut microbiota and the neonate host.
Milk provides a wealth of bioactive factors that shape the composition and function of the developing neonate intestinal microbiota. Understanding how human milk orchestrates this bacterial enrichment is critical to our comprehension of infant health and development but also provides a useful model for diet-based manipulation of the gut microbiota. Work in my lab seeks to annotate specific host factors provided in milk that influence bacterial enrichment in the neonate gut and examine how different enriched (or introduced) commensal bacteria influence host health. The goal of this work is to identify genetic and ecological factors that drive beneficial gut microbiota functions in neonates.